Quick Answer: The ocean is salty because rainwater dissolves minerals from rocks on land and carries them to the sea, while hydrothermal vents on the seafloor add more dissolved salts. Over geological time, these salts accumulate in the ocean because water evaporates but the salt stays behind, creating the salty seawater we know today.
Have you ever wondered why a sip of ocean water tastes so incredibly salty while the rivers that feed into it are perfectly fresh? This fascinating puzzle reveals one of nature’s most interesting processes. The ocean’s saltiness tells a story that spans our entire planet – from raindrops hitting mountain peaks to volcanic vents deep on the seafloor. Understanding why the ocean is salty opens a window into how our planet’s water cycle works and why life on Earth depends on this perfectly balanced system.
The Straight Scoop on Sea Saltiness
Ocean salinity measures how much salt dissolves in seawater, and the numbers might surprise you. According to NOAA, the average salinity of ocean water sits at about 35 parts per thousand – that’s 3.5% by weight. Put simply, every liter of seawater contains approximately 35 grams of salt.

But what exactly makes up this salty mixture? The sea water composition consists mainly of familiar table salt components:
- Sodium and chloride ions: These make up around 85% of all dissolved salts in the ocean
- Magnesium and sulfate ions: Contributing roughly another 10%
- Other minerals: Including calcium, potassium, and trace elements making up the remaining 5%
These ions remain dissolved because they’re highly soluble, and what makes water so special is its incredible ability to act as nature’s universal solvent. Water molecules can surround and separate these charged particles, keeping them suspended in solution.
Primary Salt Sources
Where does the salt in the ocean come from? The answer involves two main sources that have been working together for countless years to create our salty seas.
Land Runoff and Rock Weathering
The primary source starts with something as simple as rain. Rainwater contains dissolved carbon dioxide from the atmosphere, making it slightly acidic. When this acidic rainwater hits rocks on land, it begins a process called rock erosion. The water slowly dissolves minerals from the rocks, releasing ions like sodium and chloride.
These dissolved minerals then travel through streams and rivers as land runoff, eventually reaching the ocean. This process happens continuously around the globe, with every rainstorm contributing to the ocean’s salt content.

Hydrothermal Vents and Seafloor Activity
The second major source comes from deep beneath the waves. Hydrothermal vents on the ocean floor act like underwater geysers, spewing mineral-rich fluids heated by magma from Earth’s crust. These underwater volcanoes release metals and salts directly into seawater.
The role of hydrothermal vents in ocean salinity is significant because they add different types of minerals than land sources provide. According to the Natural History Museum, this seafloor activity contributes essential minerals that help maintain the ocean’s chemical balance.
Why Salts Accumulate in Oceans
Understanding how did the oceans get so salty requires thinking about the ocean as a giant collection basin. The process of salt accumulating in the ocean works like this:
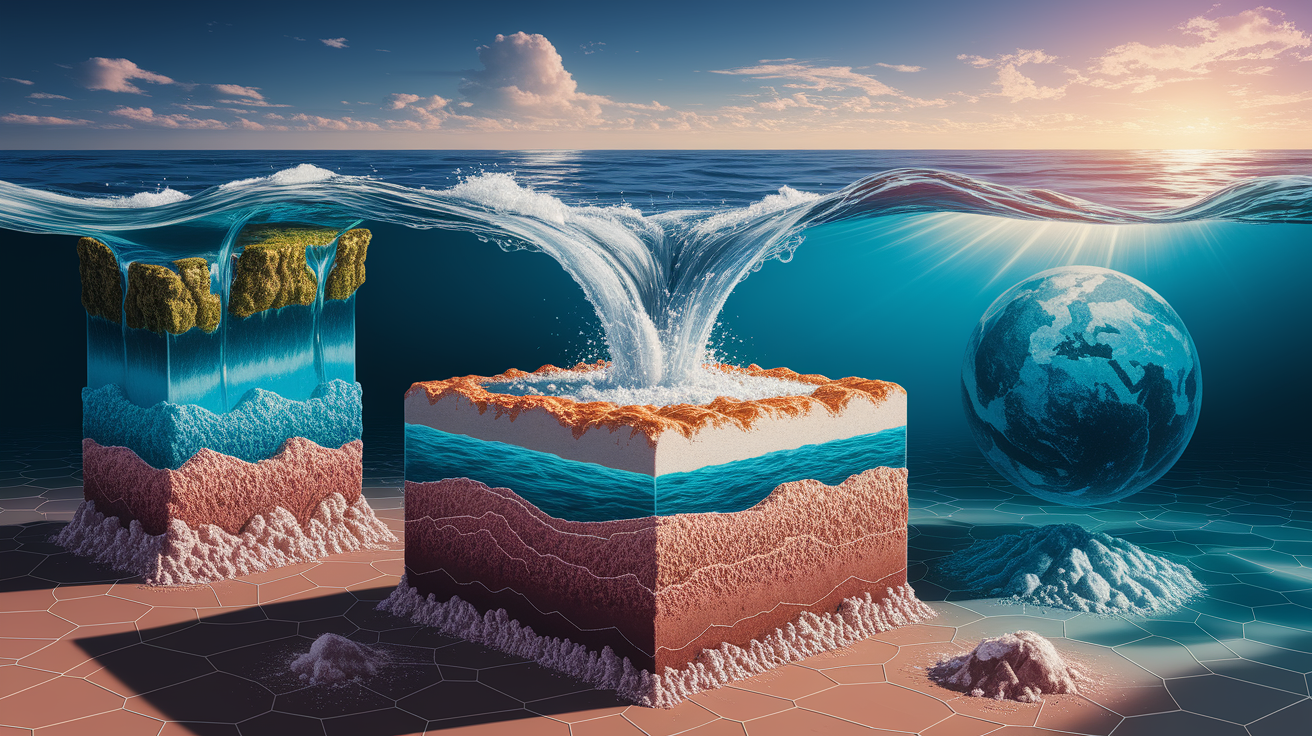
- Continuous Input: Rivers constantly deliver dissolved minerals to the ocean
- Evaporation Concentration: Water evaporates from the surface to form clouds, but salt cannot evaporate
- Salt Remains Behind: As the water cycle continues, fresh water leaves but dissolved salts stay
- Gradual Buildup: Over geological time, salt concentration increases steadily
This concentration process explains why the ocean acts like nature’s salt shaker. Every time water evaporates and returns as fresh rainwater, the remaining seawater becomes slightly more concentrated with dissolved minerals.
The ocean is essentially a closed basin where salts check in but never check out – they accumulate over vast periods while water continuously cycles through evaporation and precipitation.
Rivers vs. Oceans: Why Freshwater Stays Fresh
One of the most common questions people ask is: why are rivers fresh but the ocean salty? The answer lies in the fundamental difference between flowing and static water systems.
According to NOAA, rivers remain fresh because:
- Constant Flow: Rivers continuously flush out dissolved minerals before they can accumulate
- Regular Dilution: Rainfall constantly replenishes rivers with fresh water
- Low Concentration: The minerals carried from land sources remain at relatively low concentrations
- No Evaporation Concentration: Rivers don’t sit still long enough for evaporation to concentrate their mineral content
The ocean, in contrast, acts as the final destination for all these dissolved minerals. It’s a closed system where water leaves through evaporation, but the salt stays behind. This creates a natural concentrating effect that rivers simply don’t experience.

Think of it like adding a pinch of salt to a flowing stream versus adding that same salt to a puddle that slowly dries up. The stream carries the salt away, while the puddle becomes increasingly salty as water evaporates.
Salinity Swings Around the Globe
Ocean salinity isn’t uniform across the planet. Regional variations create fascinating differences in salt concentration based on local conditions.
High Salinity Regions
Areas with intense evaporation develop higher salt concentrations:
- Red Sea: High evaporation rates create elevated salinity levels
- Mediterranean Sea: Limited freshwater input combined with high evaporation
- Persian Gulf: Hot, dry climate leads to concentrated seawater
Low Salinity Areas
Regions receiving substantial freshwater input show lower salinity:
- Baltic Sea: Heavy rainfall and river input dilute salt content
- Arctic Ocean: Melting sea ice adds fresh water
- River Mouths: Where major rivers meet the ocean, creating brackish water
Extreme Cases
Some bodies of water take salinity to extremes. The Dead Sea demonstrates why the Dead Sea is saltier than the ocean – it’s an enclosed basin where evaporation greatly exceeds freshwater input, creating hypersaline conditions nearly ten times saltier than typical seawater.
Final Drop: Why Ocean Saltiness Matters
Ocean salinity plays crucial roles in our planet’s systems that extend far beyond just making seawater taste salty.
Ocean Circulation and Climate
Higher salinity increases water density, affecting how ocean currents flow. These currents transport heat around the globe, influencing weather patterns and climate systems. The salt balance helps drive the circulation patterns that keep our planet’s temperature relatively stable.
Marine Life and Ecosystems
Ocean chemistry depends on the right balance of dissolved minerals. Marine organisms have adapted to specific salinity levels, and changes can significantly impact marine ecosystems. The dissolved salts also make water a better electrical conductor, which affects how sea creatures navigate and communicate.
Global Water Cycle
The hydrologic cycle relies on the difference between salty ocean water and fresh precipitation. This system provides the fresh water that sustains life on land while maintaining the ocean’s chemical stability.
Understanding why the ocean is salty reveals the intricate connections between land, sea, and sky. From raindrops dissolving mountain minerals to deep-sea vents adding their chemical contributions, our salty oceans represent billions of years of planetary processes working together. This remarkable system continues today, maintaining the delicate balance that makes life on Earth possible.













